Talk:Alkaline battery
| This It is of interest to the following WikiProjects: | ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
Zinc powder? Incorrect?[edit]
- "In an alkaline battery, the anode (negative terminal) is made of zinc powder (which allows more surface area for increased rate of reaction therefore increased electron flow) and the cathode (positive terminal) is composed of manganese dioxide."
I just opened a big stack of them, the zinc is the solid outer casing, cathode. Then the manganese powder, then the carbon rod, anode button.
I'm attempting to make electroluminescent & glow in the dark materials. Zinc casing dissolved in sulphuric, reduced to sulphide using sulphur from the pet shop. Manganese reduced with the carbon electrode (coke) method to manganese. Dope the sulphide with a little manganese, suspend in some form of resin, spin, dry, illuminate in an electrostatic field. —Preceding unsigned comment added by 82.24.47.178 (talk) 15:47, 23 September 2010 (UTC)
- "A cylindrical cell is contained in a drawn steel can, which is the cathode current collector."
It isn't steel. First of all, I can tear it with my hands. Secondly, I can melt it without too much difficulty. Thirdly, it reacts with sulphuric a lot quicker than iron.
- "The paste may be pressed into the can or deposited as pre-molded rings."
Is this some sort of new generation battery? Because all the, new, ones I've opened don't look like this inside, at all. It's just casing, powder, rod.
- "The separator is made of a non-woven layer of cellulose or a synthetic polymer. The separator must conduct ions and remain stable in the highly alkaline electrolyte solution."
All the ones I've opened have what looks like card / paper on the inside wall of the zinc casing, acting as the separator.
- "The anode is composed of a dispersion of zinc powder in a gel containing the potassium hydroxide electrolyte."
The anode is carbon. The cathode is zinc.
- "When describing standard AAA,AA, C, sub-C and D size cells, the anode is connected to the flat end while the cathode is connected to the end with the raised button."
That's just flat out :) wrong. The flat is the cathode, the button is the anode. Even the articles you linked to show that. In fact, the picture at the top of the article shows it.
- Are you sure you're taking apart an alkaline battery? They usually don't have the carbon rod and zinc can. Certainly when I dug out some AA cells from a flashlight that had been left in a drawer for years, I didn't find any carbon rods. The cathode is manganese dioxide, the carbon rod or nail is just a current collector and not part of the reaction. Positive terminal of a flashlight battery is the top button connected to the
nail andcathode (manganese dioxide paste), negative terminal is the bottom of the battery, which is connects to the nail and zinc anode material. --Wtshymanski (talk) 16:11, 23 September 2010 (UTC)
- Are you sure you're taking apart an alkaline battery? They usually don't have the carbon rod and zinc can. Certainly when I dug out some AA cells from a flashlight that had been left in a drawer for years, I didn't find any carbon rods. The cathode is manganese dioxide, the carbon rod or nail is just a current collector and not part of the reaction. Positive terminal of a flashlight battery is the top button connected to the
- Revised the above which was wrong. --Wtshymanski (talk) 21:48, 29 September 2010 (UTC)
simple science[edit]
There is plenty of good simple science in batteries.
- Chemistry: ion flow, membrane properties and tradeoffs, redox potential
- Physics: energy density, capacity dependancies, internal resistance,
- History: lineage
Compare and contrast with Zinc carbon battery - article needed for this, and with Lithium battery too.
Bear in mind http://en.wikipedia.org/wiki/Battery_%28electricity%29
Chemistry[edit]
The definition of anode / cathode may be confusing. Refer to http://en.wikipedia.org/wiki/Anode#Battery_or_galvanic_cell_anode for further discussion on this topic. —Preceding unsigned comment added by 134.134.136.2 (talk) 18:05, 16 May 2008 (UTC)
An expert needs to verify this, but I think that one of the reasons for the zinc (in the form of a powder) making up the cathode is to minimize the self-discharge rate of the cell. A solid cathode immersed in an electrolyte has surface areas of different electronegativity, in part due to differing concentrations of contaminants in the zinc, so the more-electronegative areas self-discharge (corrode) and the electrons flow (through the solid electrode) to the less-electronative areas, which electrolyze hydrogen gas. This amounts to self-discharge of the battery. Forming the cathode into a powder minimizes this effect. Example United States Patent 4136236. Jamesdbell8 (talk) 03:31, 18 July 2012 (UTC)
Voltage[edit]
Added "approximately" to voltage. An alkaline cell produces 1.58 volts. I'll have to look up carbon-zinc, don't know it off the top of my head. Rsduhamel 07:16, 28 Dec 2004 (UTC)
temperature limitations?[edit]
What are the temperature limitations on alkaline batteries? What happens when you exceed them? - Brewthatistrue 22:09, 23 Jun 2005 (UTC)
Goes boom and expells murcury gas...read the article...Kr5t 22:48, 21 May 2006 (UTC)
- Alkaline cells have no added mercury; for all practical purposes, you can consider them to be essentially free from mercury. Please check your sources. chami 16:53, 28 October 2022 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
Where does it say anything about mercury gas? --Newton21989 01:55, 4 April 2007 (UTC)
Scroll down. --User:HaxOr|HaxOr <% =now %> —Preceding unsigned comment added by 75.162.44.48 (talk) 02:15, 25 April 2008 (UTC)
Usage[edit]
Alkaline batteries are preferred for voltage sensitive electronic devices. The output voltage does not drop until totally drained of useful energy. Carbon-Zinc cell output voltage will consistently drop as energy is used which will result in erratic low voltage operation of electronic equipment, hence the alkaline requirement on most electronic equipment.
62 words ~ WOW[edit]
"Once a leak has formed due to corrosion of the outer steel shell, potassium hydroxide absorbs carbon dioxide from the air to form a feathery crystalline structure of potassium carbonate that grows and spreads out from the battery over time, following along metal electrodes to circuit boards where it commences oxidation of copper tracks and other components, leading to permanent circuitry damage."
This has to certainly be the longest run-on sentence on the whole Internet. — Preceding unsigned comment added by 2606:A000:F441:9300:9C0C:153D:61C5:9958 (talk) 23:46, 26 December 2014 (UTC)
- Oh my dear sweet naive child, this isn't even CLOSE. Go for a little cruise around the interwebs and keep your eyes open. Heck, I've probably written longer myself sometime in the last week. 193.63.174.254 (talk) 16:25, 21 February 2017 (UTC)
Environmentalism[edit]
What is the impact of modern alkaline batteries on the environment? The fact that there is no longer mercury added to them helps, being a heavy metal, but are there any other traces of heavy metals in alkaline batteries?
There still is mercury...Kr5t 22:48, 21 May 2006 (UTC).
- However at the site: www. duracell.com it reads that they have not had mercury in their batteries since they voluntarily discontinued producing them with it in 1993. —The preceding unsigned comment was added by 67.142.130.40 (talk • contribs). 18 nov 2006
- I'm not very familiar with battery recycling, but it seems that this would be something good to add. Maybe some guidelines on proper battery disposal too. Update: I added a link on battery recycling which I thought was informative. —The preceding unsigned comment was added by 71.117.254.131 (talk • contribs). 4 Aug 2006
Can anyone put these compunds into english please?
Self recharging[edit]
After letting used batteries sit for a while they seem to partially recharge (although it doesn't last long). How does this happen? What are the results, etc? Is it dangerous to use a battery up like this? Thanks --32.97.110.142 17:18, 7 March 2007 (UTC)
- The battery doesn't really recharge. Here's an explanation for this curious effect. I just noticed this as well when using a (cheap) alkaline battery in a 1xAAA high drain flashlight. NiMH batteries don't sag as quickly, and they do not noticeably recover either. Aragorn2 (talk) 12:31, 15 April 2009 (UTC)
White crystals[edit]
Sometimes alkaline batteries leak, a clear fluid. But sometimes white crystal powder forms at one end. It seems to corrode contacts. What exactly is this white material, what exactly causes it, how can it be avoided, and what contact materials are most resistant to corrosion? -69.87.193.60 17:27, 22 March 2007 (UTC)
- the liquid is KOH, chemically known as potassium hydroxide, an extremely corrosive liquid (solution). slowly it absorbs carbon dioxide from the air and forms potassium carbonate, similar to soda ash, with the chemical formula K2CO3. This is less corrosive but you should still handle it with care. chami 17:00, 28 October 2022 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
- I've had this happen many times while recharging alkaline batteries. This is potassium hydroxide and in my experience, has not seemed to cause any damage to myself or objects it comes in contact with. My advice, to be safe, is just wipe it up with a paper towel, or likewise and throw the battery (It's no good after it pops.) and the towel into a trash container.
The liquid is Potassium Hydroxide solution which is corrosive. For a description of the powder see similar comments here Talk:Nickel–cadmium_battery#White_powder_-_cell_leakage - the powder is potassium carbonate, which is also corrosive. John a s (talk) 00:17, 18 September 2011 (UTC)
Recycling[edit]
Information is needed about recycling and disposal practices for alkaline batteries, in various countries around the world. -69.87.193.60 17:27, 22 March 2007 (UTC)
Capacity and Current[edit]
There seems to be a contradiction between the Capacity section saying that loads of 1000mA can reduce the energy content due to internal heating and the Current section saying that a AA battery can deliver 1000 mA without significant internal heating. Am I missing something? --Nathan24601 (talk) 20:50, 24 February 2008 (UTC)
Anode or cathode[edit]
Isn't manganese dioxide the cathode? It says the anode.. but manganese dioxide is gaining electrons and is the cathode in the battery? Am I wrong? —Preceding unsigned comment added by 88.88.108.79 (talk) 14:34, 2 May 2011 (UTC)
History[edit]
When were ordinary AA/AAA/C/D batteries first commonly sold? -69.87.203.130 (talk) 00:43, 18 June 2008 (UTC)
Rechargeable Alkaline Batteries[edit]
I noticed there is not a separate article on these, only an article on recharging standard disposable alkaline batteries. In what ways are rechargeable alkaline batteries different than disposable alkaline batteries? Obviously, other than the ability to recharge. Is the difference in the battery, or in the charger, or in both? When Rayovac released the Renewal line of rechargeable alkaline batteries in 1993 I had a suspicion they had simply created a "smart" charger that could safely recharge any alkaline battery and that the batteries themselves were nothing more than overpriced relabeled versions of their disposable line since they were packaged fully charged. I could be completely wrong on this since I was only 12 at the time and also thought connecting two 9-volt batteries together made a convenient hand warmer. I was a very lucky kid who had great success in recharging my used disposable alkaline batteries in my Ni-Cad charger. By success I mean I never hurt myself or my Game Boy, not that the recharged battery ever lasted very long.
By the way, this is my very first post in Wikipedia so I apologize if I have done so incorrectly. Rlsaine (talk) 18:40, 3 July 2008 (UTC)
- There is now a separate article, see Rechargeable alkaline battery. Biscuittin (talk) 18:55, 13 October 2009 (UTC)
It's just a stronger case and slightly different chemicals. Standard cells can be recharged safely with a smart enough charger. (Though I still wouldn't recommend it) — Preceding unsigned comment added by 67.142.175.21 (talk) 00:40, 20 February 2013 (UTC)
Category[edit]
I noticed that Twinzor reverted my category removal - see this edit. However, I removed the category from the article because Category:Disposable batteries is actually a subcategory of Category:Electric batteries. Thus I was just following the categorisation editing guidelines. Point 3 suggests that "Usually, articles should not be in both a category and its subcategory." Thus I have reremoved the category, as per the guideline. - Tbsdy lives (talk) 07:19, 30 August 2008 (UTC)
- I apologize for that. My edit was due to me not notising disposable batteries was a category of electric batteries, and also because I misunderstood Tbsdy lives's edit summary "remove category - articles should only be part of one category, and only then should be in the narrowest one". Seeing the situation now with the categories as it is and what Tbsdy meant in the summary, I completely agree with his edit. I'll remind myself to double check things before I make such edits in the future. :) --Twinzor (talk) 20:28, 31 August 2008 (UTC)
- No probs :-) I do find it somewhat hard to make it clear what I'm doing in just one edit summary line. - Tbsdy lives (talk) 05:46, 4 September 2008 (UTC)
Pronounciation[edit]
There seems to be a dispute on whether "A" or "An" should be used with AA batteries. The Wikipedia article on AA battery gives Double A as the only pronounciation, altough I've certainly heard it pronounced as just A A as well. However, since the relevant article give the pronounciation double A and uses "A AA", weird as it seems, I think we should use the same style for consistency. Thoughts? — Twinzor Say hi! 18:47, 30 January 2009 (UTC)
- They all work, though I haven't heard anyone regularly violate the typical grammatical rules regarding "a"/"an" when talking about them. EG, "a double-A battery" vs "an A-A battery". Sounding out individual letters or numbers, vs using double-, triple- etc (or in the case of road/phone numbers, saying them as compound numbers at least up into the units-thousand (and maybe tens-thousand in languages where that has its own word) instead of discrete digits) is a matter of personal style rather than any kind of strict set rule. I think someone saying or writing "a AA battery" needs at least a little bit of remedial English tuition, though :) 193.63.174.254 (talk) 16:29, 21 February 2017 (UTC)
Leaking ... why does it leak out? Internal cell pressure?[edit]
Generally most batteries are sealed or have one-way gas vents, so it is unclear to me how the acid manages to leak out without there being another hole somewhere else for air to enter, in place of the leaking fluid. Most batteries are in plastic cases so I don't see how absorption out of the battery could be occurring.
What causes the fluid to seep out?
DMahalko (talk) 02:58, 5 April 2009 (UTC)
- Alkali, not acid. The "Handbook of Batteries" says that zinc in the battery will dissociate water and make hydrogen gas, also that the products of the reactions change in density. Either one of these would increase pressure inside a cell and make it bulge and leak. Simple gravity probably accounts for the rest once a hole gets rusted through the sheet metal jacket of the cell. --Wtshymanski (talk) 17:12, 5 April 2009 (UTC)
"potassium carbonate that grows and spreads out from the battery over time, following along metal electrodes to circuit boards where it commences oxidation of copper tracks" The article says that potassium carbonate is formed, which then reacts with the copper tracing. Can anyone provide a balanced chemical reaction for this process? Is it a redox reaction where copper is oxidized and forms copper carbonate and potassium metal? I cannot find any evidence of this reaction. Lhammer610 (talk) 16:08, 14 February 2016 (UTC) [1]
built-in testers[edit]
Does anyone else remember these from the 90s? A little strip on the side that would glow when a button was pressed to indicate the remaining capacity. Definitely a drain on the cell as they would get hot. If anyone can come up with a source I think it would be a nice addition to the article. Btyner (talk) 14:09, 22 August 2009 (UTC)
- They never went away. In fact I bought a pack of duracells recently that had a newer type (with four blocks ranging from green to red to indicate quarters of full charge remaining) prominently displayed on the side, and although it's certainly not been a feature of every single battery I've ever bought, it's certainly made an appearance regularly down the years. I'd suggest the Duracell or Energiser companies' websites as a good place to start for reference, given that they're the main, possibly only brands to have used the idea, at least to my knowledge. Possible that they were introduced in the 90s, with some fanfare, but are now just "one of those things"? One notable feature of the modern iteration does seem to be that they're MUCH easier to operate now - the original type required so much pressure that it felt like you were going to do yourself an injury, and after testing a half dozen AAs your fingertips tended to be numb for a minute then sore for a while longer... these ones do require a bit of pressure (to avoid accidentally operating it in a pocket, or inside a device), but nowhere near as much and not even uncomfortable let alone painful.
- ((Now, if only one of the rechargeable battery manufacturers would incorporate the same idea into THEIR products, it would make life SO much easier when having to put a set of NiMHs into some infrequently used device, out of a drawer with a couple dozen of them in... I mean, battery testers and multimeters exist, but they're all kinda fiddly...)) 193.63.174.254 (talk) 16:36, 21 February 2017 (UTC)
- Unfortunately, they would be useless on a NiMH battery. The internal resistance of primary batteries rises as the insoluble reaction products are produced. This imposes an increasing limit on the current that can be produced which the battery tester can measure. There are no such products in a NiMH battery and nothing to limit the current as it discharges. These batteries maintain a near constant voltage and internal resistance during their discharge cycle and then, more or less, die suddenly as the reactants are consumed. 86.134.23.220 (talk) 13:38, 23 March 2021 (UTC)
Battery names[edit]
This is not a re-naming proposal but a suggestion that more explanation is needed. The term "alkaline battery" in everyday use means a primary cell of the zinc/manganese/alkali type. However, secondary cells of the nickel/iron/alkali and nickel/cadmium/alkali types are also alkaline batteries. Can we make this clear without generating confusion? Biscuittin (talk) 19:09, 13 October 2009 (UTC)
- If anyone routinely called anything other than a zinc/manganese dioxide/ potassium hydroxide system an "alkaline battery", maybe there would be a reason to provide more explanation. Theoretically confusing, in practice, everyone knows which is which. --Wtshymanski (talk) 04:02, 29 November 2009 (UTC)
- I agree more explanation is required. Some books list many non-zinc types as 'alkaline' and this article has a picture of Nickel–iron battery in the history section. - Rod57 (talk) 02:01, 11 November 2014 (UTC)
- Alkaline cells and batteries are those marketed as such (they rarely display their internal formulation on the packaging...) and with high-pH, typically hydroxide-based "dry" paste electrolytes, regardless of whether they're rechargeable. Anyone who calls a different type of cell/battery "alkaline" despite it being of a different composition, or indeed because it's primary rather than secondary (and ignore the fact that you tend to get (implicitly primary) "alkaline" and (obviously secondary) "rechargeable alkaline" offered for sale), is merely doing so out of laziness or ignorance and ideally needs to be gently corrected.
- It IS a bit of a problem, and I think trying to claw back some mental segregation is the only real reason the big manufacturers still bother highlighting just how much more capacious their products are versus "normal" zinc-carbon (acid) types - which despite that epithet, most people probably haven't bought a pack of in the last 20 years unless they've been duped at a discount store... which is also why it's a "bit" of a problem, but certainly not a big one. Maybe more annoying that they also get confused with the high power, high capacity Lithium cells that are packaged into regular AA and AAA shapes but have significantly better performance and longevity in high-drain devices...
- Either way, the casual misuse of the term by people who don't really know what they're talking about shouldn't be something that overly concerns us when editing the text of an encyclopaedic work. The text as it stands, including the lede, clarifies what's being discussed and separates it from the other types quite nicely as far as I'm concerned. If folk can't be bothered reading four or five lines of text, even though they're on an encyclopaedia site, that's not our problem. 193.63.174.254 (talk) 16:45, 21 February 2017 (UTC)
Unified layout[edit]
On other articles about battery tech (like: Lithium-ion_battery and Zinc_air). There is a table about properties like energy/weight energy/size, etc. After searching through this article I was unable to find those values. If someone (including me of course) finds a good source, please add :) Dorit82 (talk) 03:41, 29 November 2009 (UTC)
- >was looking for them on the article page
- >came to discussion page to see if anyone had already asked the question, whether there was an answer if so, and to ask it myself if not
- ...
- >question is already here
- >...right at the bottom of the page, so it's literally the last thing I come to
- >post date, seven and a half years ago
- > . . . . . no answers . . . . .
- DAMMIT
- .
- Guess I'll have to see if I can find anything out then. Too bad "borrowing" a ten-pack of Energiser Industrial AAs from work, hooking them up in turn to a voltmeter, ammeter and a sample load of *insert_number* ohms in front of a camera set to time-lapse mode, then plunking the results into a spreadsheet and coming up with an mean or 2sd min-max example figure for these entirely average (not terrible, but certainly not best of breed either, just good-enough and very affordable) alkaline cells would end up counting as Original Research :-/ ...
- ...if I do that and put it on a webpage, can someone else "find" it and cite it? ;) 193.63.174.254 (talk) 16:51, 21 February 2017 (UTC)
- (actually, thinking about it, I'm sure I've got that idea from seeing some existing website where some random obsessive has done exactly that with examples of pretty much every available type of alkaline, lithium, zinc-carbon and rechargeable battery going, along with comparison tests of various Nickel-** and Lithium-Ion chargers... I'll have to see if I can google it up again. I don't think they bothered including a range in their results, but given the huge breadth of samples available to browse it shouldn't be too hard to just find the best and the worst... Not sure what to do for capacity per kilo, or per dollar, but the internal volume is more or less fixed for each cell size, the weight should be within a fairly limited range both within and between each particular chemistry, and the price can either be googled or just fudged, as the cost of primary cells doesn't vary TOO much year on year, at least at retail; the biggest determinant is brand, pack size, and what retailer you're buying from. Wholesale might be a different kettle...) 193.63.174.254 (talk) 16:53, 21 February 2017 (UTC)
Move[edit]
- The following is a closed discussion of the proposal. Please do not modify it. Subsequent comments should be made in a new section on the talk page. No further edits should be made to this section.
The result of the proposal was consensus against move
Alkaline battery → Alkaline electrochemical cell — The electrochemical battery is simply a pair of electrochemical cells. this article deals around how this type of cell works. —Preceding unsigned comment added by 81.245.90.148 (talk • contribs) 11:58, 9 October 2009
- Oppose. Just wrong. This is one of several *highly dubious* renamings proposed by this IP address. There's no need to give encyclopedia articles obscure names when the common name is accurate and sufficient. --Wtshymanski (talk) 14:33, 9 October 2009 (UTC)
- Oppose, this is at the common name and should remain. ~~ GB fan ~~ talk 15:19, 9 October 2009 (UTC)
- Strong Oppose and suggest speedy close (is that possible?). This is not what the general public would be looking for. HumphreyW (talk) 15:25, 9 October 2009 (UTC)
- Oppose WP:COMMONNAME 76.66.197.30 (talk) 16:12, 9 October 2009 (UTC)
- Oppose The term "battery" is almost always used by consumers, battery manufacturers, etc. TJ Spyke 17:29, 9 October 2009 (UTC)
Move discussion in process[edit]
There is a move discussion in progress on Talk:Lead-acid battery which affects this page. Please participate on that page and not in this talk page section. Thank you. —RFC bot 00:00, 11 October 2009 (UTC)
- The above discussion is preserved as an archive of the proposal. Please do not modify it. Subsequent comments should be made in a new section on this talk page. No further edits should be made to this section.
Anode and cathode[edit]
Quoting the battery handbook for reference:
Powdered
zinc is used for the anode to provide a large surface area for high-rate capability (that is, to reduce current density) and to distribute solid and liquid phases more homogeneously (to minimize mass-transport polarization of reactant and product). On discharge, the manganese dioxide cathode undergoes at first a one-electron reduction
to the oxyhydroxide...
(David Linden, Thomas B. Reddy (ed). Handbook Of Batteries 3rd Edition. McGraw-Hill, New York, 2002 ISBN 0-07-135978-8 Chapter 10, page 10.3-10.4 )
because I sometimes don't have it handy when I need to revert the vandals. --Wtshymanski (talk) 22:49, 9 December 2009 (UTC)
leaking[edit]
Hi, when the pottasium hydroxide is leaking out it is not forming crystals. It is reacting with CO2 from air and forms Potassium carbonate (: 2KOH + CO2 → K2CO3 + H2O ). It is also corrosive and the reaction takes some time so the pottasium hydroxide is destroying surroundings as well.
I'm not so much advanced in english so i don't want to edit directly. —Preceding unsigned comment added by 90.176.57.105 (talk) 18:03, 21 March 2010 (UTC)
Assessment[edit]
A fresh new tag calls this "start" class. Let's look at Wikipedia:WikiProject Technology/Assessment
B-ClassThe article meets the following six criteria:
- 1. It is suitably referenced, and all major points are appropriately cited.
Nine references cited, most paragraphs have at least one reference except for some very general statements which are hardly controversial (bigger batteries have more capacity than smaller batteries, and the like).
- 2. It reasonably covers the topic, and does not contain major omissions or inaccuracies.
It has history, chemistry, capacity, recharging and disposal sections. Each section could be somewhat expanded, but there's no major ommissions.
- 3. It has a defined structure, including a lead section and one or more sections of content.
Duh.
- 4. It is free from major grammatical errors.
Duh. "Word" just now only quarreled about a "that/which" substitution which I refuse to change merely on the advice of Word.
- 5. It contains appropriate supporting materials, such as an infobox, images, or diagrams.
Infoboxes are a pox. We've got sufficient diagrams and pictures.
- 6. It is written from a neutral point of view
Duh. It's about a battery, even Wikieditors don't cry "jihad!" over batteries.
- B-Class articles are selected by individual assessors.
I think it should be mandatory that any drive-by tagger give us a point by point evaluation against the project's criteria. I recommend that any article class evaluations not accompanied by a point by point evaluation should be removed as pointless Wikitwiddling. We *love* tags in this project because it's so much easier to tag something than to FIX it. --Wtshymanski (talk) 16:00, 28 January 2011 (UTC)
- Agreed, many article assessments seem more like meta-editing, it lets people feel like they are contributing something, without really doing anything. Many are really no better than random vandalism. Are you done with your TPS reports? :) DMahalko (talk) 18:28, 28 January 2011 (UTC)
Alkaline reserve battery?[edit]
Apparently somewhere around 1967-1971 or so, the Mallory Battery Company (now Duracell) offered an alkaline reserve battery as a consumer product. This could be kept on the shelf for years, then turning a screw in the top of the battery would break an internal electrolyte resevoir and make it ready to use. Aside from a breathless press release in a 1967 "Popular Science" I can't find much more about these; they didn't make it into the Linden battery handbook. Has anyone seen any more on this reserve battery? --Wtshymanski (talk) 18:51, 16 March 2012 (UTC)
- I'll have a gander around my school’s archives, see if any more mentions come up. I think it probably never went anywhere though.--Lead holder (talk) 09:36, 17 March 2012 (UTC)
graph of resistance and voltage as discharging[edit]
Graph(s) would be very helpful. - Rod57 (talk) 02:07, 11 November 2014 (UTC)
External links modified[edit]
Hello fellow Wikipedians,
I have just added archive links to 2 external links on Alkaline battery. Please take a moment to review my edit. If necessary, add {{cbignore}} after the link to keep me from modifying it. Alternatively, you can add {{nobots|deny=InternetArchiveBot}} to keep me off the page altogether. I made the following changes:
- Added archive https://web.archive.org/20120325171702/http://www.inobat.ch/fileadmin/user_upload/pdf_09/Absatz_Statistik_2008.pdf to http://www.inobat.ch/fileadmin/user_upload/pdf_09/Absatz_Statistik_2008.pdf
- Added archive https://web.archive.org/20120321012709/http://www.epbaeurope.net/statistics.html to http://www.epbaeurope.net/statistics.html
When you have finished reviewing my changes, please set the checked parameter below to true to let others know.
![]() An editor has reviewed this edit and fixed any errors that were found.
An editor has reviewed this edit and fixed any errors that were found.
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—cyberbot IITalk to my owner:Online 22:41, 7 January 2016 (UTC)
External links modified[edit]
Hello fellow Wikipedians,
I have just modified one external link on Alkaline battery. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive http://web.archive.org/web/20060312193002/http://www.alliedelec.com/Images/Products/Datasheets/BM/DURACELL/Duracell_Power-Products_7370520.pdf to http://www.alliedelec.com/Images/Products/Datasheets/BM/DURACELL/Duracell_Power-Products_7370520.pdf
- Added
{{dead link}}tag to http://www.afrotechmods.com/reallycheap/batteries/batts.htm
When you have finished reviewing my changes, please set the checked parameter below to true or failed to let others know (documentation at {{Sourcecheck}}).
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 10:35, 21 July 2016 (UTC)
Leaks and corrosion[edit]
The current section about LEAKS is interesting and rather detailed, but lacks any sources. What proof is there that mixing batteries or not replacing them all at once makes leaking more likely? What would be the exact scientific cause if this is true? Is there a significant difference between different brands of alkaline batteries with regard to leaking and corrosion? There is currently a statement that all batteries will eventually leak; this seems at least somewhat misleading; many common alk batteries can often last for decades (with little or no usage) and not leak. Please add good appropriate sources to the article.-71.174.180.38 (talk) 15:17, 2 September 2016 (UTC)
Leakage cause details:
- michaelbluejay.com/batteries/disposable.html
- "The reason why mixing fresh/used or alkaline/other batteries together increases the chance of leaking is that one battery will likely become exhausted first, and it can go to very low or even negative voltage levels, and low or negative voltage greatly increases the chances of leakage. (Energizer, PDF) data.energizer.com/PDFs/non-rechargeable_FAQ.pdf
- "If an alkaline leaked, clean up as much of the solid or liquid matter as you can with tissue paper and/or cotton swabs. After that, use cotton swabs with vinegar or lemon juice. The acid will neutralize the leaked alkaline material to prevent further corrosion. An old toothbrush or tiny wire brush might help. (If using one of those, wear safety glasses, so you don't flick the solution into your eyes.)"
-71.174.180.38 (talk) 15:34, 2 September 2016 (UTC)
pH[edit]
Why don't I find an explanation in the text why alkaline batteries are called alkaline? I know that a pH>7 equals alkaline, however, which part is alkaline? And is there a more accurate number that tells how alkaline it is? Does the degree of alkalinity change over time of discharge? It probably is very basic information but it is lacking. --VanBuren (talk) 11:59, 27 March 2017 (UTC)
- Fourth line of the lead says "The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide, instead of the acidic ammonium chloride or zinc chloride electrolyte of the zinc-carbon batteries. " I've never seen anything where the pH of the electrolyte is correlated with the condition of the battery. Since it's a saturated solution and since the electrolyte isn't a reactant in these batteries (or not intended as the reactant, anyway), I wouldn't have thought to look for a correlation between pH and battery charge. --Wtshymanski (talk) 19:26, 27 March 2017 (UTC)
- Thanks, did not see that line with explanation. --VanBuren (talk) 07:01, 28 March 2017 (UTC)
- The alkaline battery gets its name because it has an alkaline electrolyte of potassium hydroxide- This is true.
- Since it's a saturated solution- false. A saturated solution of KOH will react with Zn and produce H2 gas. Please check your source.
- since the electrolyte isn't a reactant- false. The chemical reaction you mentioned is correct and OH- ions are consumed (they are released at a different place) in the reaction. In fact the ZnO is never produced but we actually get potassium zincate (there is an article on sodium zincate in wikipedia). For every mole of Zinc metal, approx 2-4 moles of KOH are consumed. chami 17:28, 28 October 2022 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
External links modified[edit]
Hello fellow Wikipedians,
I have just modified 3 external links on Alkaline battery. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Corrected formatting/usage for http://www.baj.or.jp/e/statistics/02.php
- Added archive https://web.archive.org/web/20131008081530/http://www.epbaeurope.net/090607_2006_Oct.pdf to http://www.epbaeurope.net/090607_2006_Oct.pdf
- Added archive https://web.archive.org/web/20121006054936/http://www.rawmaterials.com/faq/ to http://www.rawmaterials.com/faq/
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 18 January 2022).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 00:24, 2 July 2017 (UTC)
Unscientific voltage table[edit]
The "33 Ω" row and the table caption "Open circuit, zero-load voltage and voltage for a 33 Ω load (330 mW) vs capacity" are nonsense unless cell capacity or discharge rate (current/capacity) used is stated. — Preceding unsigned comment added by 83.209.123.250 (talk) 22:56, 23 February 2018 (UTC)
- I've added the battery size (AA), and changed the 33 ohm to 330 mW. Without the full context the 33 ohm made no sense (the battery was powering a boost converter, and the 33 ohm load was connected to the output of the converter, not to the battery). At 1.5V, a 33 ohm load would only draw 68mW. Prevalence 19:59, 12 May 2019 (UTC)
Remove Edison as inventor[edit]
Edison didn’t invent the NiFe battery, this was invented by Jungner as well with the first patent registered in 1897 four years prior to Edison. — Preceding unsigned comment added by 83.233.2.6 (talk) 20:31, 17 February 2020 (UTC)
Incorrect illustration.[edit]
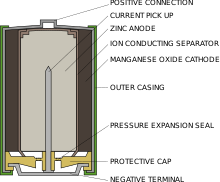
The illustration to the right is incorrect on two counts and has been removed from the article.
- It shows the cell as containing manganese oxide. This is wrong: it is manganese dioxide. Manganese oxide would be totally useless as it is one of the products of the overall reactions. This is most probably a typo and should have read 'manganese dioxide'.
- Even if labelled correctly, it shows that the manganese (di)oxide as the cathode of the cell. This is incorrect.
The Manganese dioxide takes no part in the production of electric current. It couldn't even if it wanted to because it is a complete non conductor and as such cannot conduct current into or out of the cell. All the equations are incorrect for the same reason. In any primary cell, the cathode actually takes no part in the reaction at all. It merely serves as a conductor (which manganese dioxide isn't) to allow the developed electron flow to flow back into the cell. The choice of cathode material does affect the E.M.F. of the cell because there is a potential between the cathode and the electrolyte. The manganese dioxide serves no purpose other than as a depolariser to convert the hydrogen produced at the cathode's surface into water. Indeed, if the manganese dioxide were to be removed from the cell completely, it's E.M.F. would be exactly the same, except the cell would polarise as the current would rapidly drop as the gaseous hydrogen formed at the cathode would block the current flow. This is the problem that the depolariser (manganese dioxide) is there to solve.
For some reason modern textbooks insist on combining the two separate reactions into one giving the erroneous impression that the manganese dioxide is part of the current producing reaction - it isn't.
The hydrogen oxidation reaction is separate from the electrochemical reaction and is.
2MnO2 + 2H --> Mn2O3 + H2O (Note: 2H not H2 as the hydrogen produced does not get to form a molecule before being grabbed by the MnO2)
This needs to be removed from the electrochemical equations to make them correct. 86.134.23.220 (talk) 12:21, 23 March 2021 (UTC)
- 2MnO2 + 2H --> Mn2O3 + H2O (Note: 2H not H2 as the hydrogen produced does not get to form a molecule before being grabbed by the MnO2)
- Unfortunately you are wrong; but your point #1 is appreciated. It should be MnO2
- Your above equation is wrong because you do not show from where the two protons come.
- The original equations are correct because the two electrons travel via the external circuit. The porous membrane allows transport of protons to allow charge balance. chami 17:40, 28 October 2022 (UTC) — Preceding unsigned comment added by Ck.mitra (talk • contribs)
Incorrect chemistry - should be MnOOH?[edit]
I'm not sure if this is incorrect, since I don't know all that much about electrochemistry, but I was looking into alkaline batteries and found several sources[1][2][3] suggesting that MnOOH (or HxMnO2 for the last one) rather than Mn2O3 is produced at the MnO2 electrode. I can guess that MnOOH might decay into Mn2O3, but it still seems misleading to just describe Mn2O3 being formed if that isn't what's actually going on. Can someone who has a better understanding of this than I do please check this? I don't want to just edit it when I don't have a firm understanding of what's going on, especially since I also found a source claiming both that the given equation is correct and that the cathodic reaction on discharge is "not possible to describe [...] in a simple unambiguous way"[4]. CuriousEnzyme (talk) 13:06, 1 June 2021 (UTC)
References
negative terminal) is made of zinc powder (which allows more surface area for increased rate of reaction therefore increased electron flow) and the cathode (positive terminal) is composed of manganese dioxide." I just opened a big stack of them, the zinc is the solid outer casing, cathode. Then the manganese powder, then the carbon rod, anode button. I'm attempting to make electroluminescent & glow in the dark materials. Zinc casing dissolved in sulphuric, reduced to sulphide ==
negative terminal) is made of zinc powder (which allows more surface area for increased rate of reaction therefore increased electron flow) and the cathode (positive terminal) is composed of manganese dioxide." I just opened a big stack of them, the zinc is the solid outer casing, cathode. Then the manganese powder, then the carbon rod, anode button.
I'm attempting to make electroluminescent & glow in the dark materials. Zinc casing dissolved in sulphuric, reduced to sulphide 196.188.180.145 (talk) 21:00, 27 March 2022 (UTC)
The construction section has no citations[edit]
The construction section has no citations yet makes some bold claims 2406:2D40:4092:9310:D05F:DAAD:7EC2:CD51 (talk) 09:37, 23 April 2024 (UTC)
- C-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Technology
- C-Class level-5 vital articles
- Wikipedia level-5 vital articles in Technology
- C-Class vital articles in Technology
- C-Class energy articles
- Mid-importance energy articles
- C-Class Chemistry articles
- Mid-importance Chemistry articles
- WikiProject Chemistry articles
- C-Class electronic articles
- High-importance electronic articles
- WikiProject Electronics articles
- C-Class Technology articles
- WikiProject Technology articles



