Talk:Pyrimidine
| This It is of interest to the following WikiProjects: |
||||||||||||||||||||||||
| ||||||||||||||||||||||||
Untitled[edit]
What is the derivative of Pyrimidine?
Aurora Fine Chemical is a Spam link.
The value of commercial suppliers in chemistry articles can be discussed here: http://en.wikipedia.org/wiki/User_talk:213.188.227.119
It is important to establish general guidelines. Please contribute to the discussion.
- RfC on Commercial Suppliers --Dirk Beetstra 06:35, 30 May 2006 (UTC)
Off topic[edit]
Pyrimidine itself is worth its own article. Heterocyclic ring motives are generally discussed in the wikipedia by mixing up the parent compound and lots of derivatives, that's not good. For example in the pyrimidine article there's more on DNA bases than on pyrimidine itself as a unique chemical compound. That's redundant and therefore no good, there should be a link to uracil, cytosin and so on, not more. The organic reaction that is drawn produces a substituted quinazoline, not a pyrimidine. There's no direct connection between those compounds, naphtalene synthesis is not discussed in the benzene article and isoquinoline synthesis is not discussed in the pyridine article, that's because these are different parent compounds. The article says that electrophilic aromatic substitution is harder than in the pyridine series, not true, pyrimidines are easier to halogenate at the C5-position than pyridine because the aromatization energy is lower. It is true that they are more reactive than pyridines towards nucleophilic aromatic substitution, but diazotization is a bad example because the yields in diazotization reaction are equally low in the pyridine and the pyrimidine series. —Preceding unsigned comment added by 92.206.166.42 (talk) 19:24, 19 March 2011 (UTC)
Atom Numbering[edit]
Hi! We are confused about the numbering of atoms in the pyrimidine cycle. According to the general IUPAC rules, atoms are numbered such that double bonds get the lowest numbers, which would mean that the numbers of the Nitrogen atoms are reversed compared to the diagram in this article (and also some other related diagrams, e.g. cytosine). Also, the official UPAC Gold Book at http://goldbook.iupac.org/P04958.html numbers the nitrogen atoms in reverse. However, we are no chemists so we would like to leave the final editing to someone more knowledgable about the topic. Pasky (talk) 16:00, 11 March 2012 (UTC)
Moreover, Organic Syntheses, Coll. Vol. 5, p.794 (1973); Vol. 43, p.77 (1963) Link referenced by the article also uses numbering consistent with above but not with this article. Pasky (talk) 16:12, 11 March 2012 (UTC)
- Numbering is not an issue for pyrimidine itself, the molecule has a mirror plane (through C2 and C5), so numbering in either direction gives the same result. Note that since it is aromatic, there are no real single and double bonds, so this is not the basis for numbering. Instead, the heteroatom (one of the N) is assigned 1, and numbering proceeds in a direction which results in the lowest numbers (1,3). Szaszicska (talk) 15:32, 28 March 2018 (UTC)
Possible additions[edit]
History and sources are sections that could be added to this page Katesee (talk) 20:02, 11 October 2012 (UTC)
Expanding and improving this article[edit]
A colleague and myself are planning to expand, update and polish this article in the next week or two. We plan to follow the layout of the indole article to some extent, with sections along the lines of lede/contents, then general properties and occurrence, history, synthesis/preparations, chemical reactions (the latter two sub-categorized if necessary, and also for common derivatives and analogs), applications/uses, See Also, general references, and references. For this article we will obviously need a section on pyrimidine bases in biochemistry also. If you are interested in helping out, please reply below, and let us know what you'd like to focus on. Walkerma (talk) 05:37, 30 December 2012 (UTC)
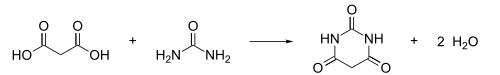
A possible synthesis of pyrimidine from hydrogenation and elimination of water of barbituric acid[edit]
Apparently, this possibility is mostly on the unsubstituted pyrimidine. Substituents on nitrogen and carbon of the ring may need to be protected before hydrogenation.
-- Mountainninja (talk) 05:22, 29 June 2015 (UTC)
- Start-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Physical sciences
- Start-Class level-5 vital articles
- Wikipedia level-5 vital articles in Physical sciences
- Start-Class vital articles in Physical sciences
- Start-Class Molecular Biology articles
- Unknown-importance Molecular Biology articles
- Start-Class MCB articles
- High-importance MCB articles
- WikiProject Molecular and Cellular Biology articles
- All WikiProject Molecular Biology articles
- Start-Class chemicals articles
- Mid-importance chemicals articles